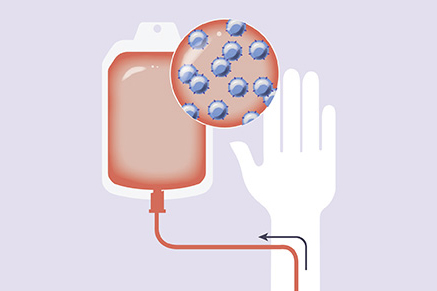
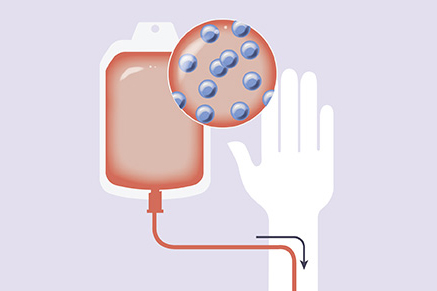
By protecting healthy blood cells, our approach to Hematopoietic Cell Transplant (HCT) expands the anti-leukemia armamentarium for patients with AML and other blood cancers.
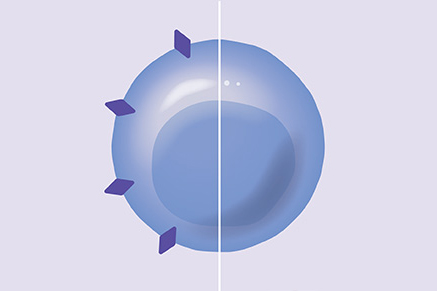
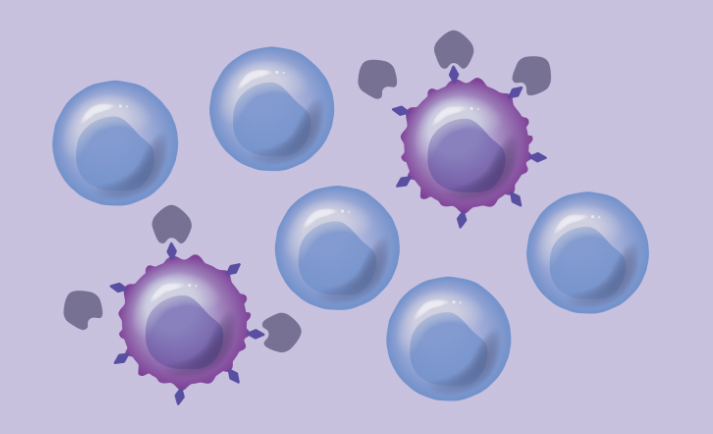
Our proprietary approach built on HSC biology, genome engineering, and Chimeric Antigen Receptor T (CAR-T) cells genetically modifies healthy donor HSCs to remove select cell surface targets that are shared between healthy blood cells and cancer cells. This approach aims to enable targeted therapies to destroy cancer cells selectively while shielding healthy blood cells, making these HSCs and their progeny resistant to targeted therapies.
Protection of healthy blood cells can expand the use of different therapeutic approaches following transplant of HSCs that have not been possible previously. By combining our engineered HSCs with targeted leukemia therapies—including Antibody Drug Conjugates (ADCs) and Chimeric Antigen Receptor T (CAR-T) cells—we hope to advance the standard of care for AML and other blood cancers.
Our clinical data has demonstrated that trem-cel in combination with Mylotarg demonstrated engraftment, shielding, broadened therapeutic window, and patient benefit.